First observed in 2003, the National Latino AIDS Awareness Day is celebrating its 20-year anniversary. NLAAD’s first campaign theme was “Open your eyes,” aiming to raise awareness of the HIV/AIDS epidemic in the Hispanic/Latinx community. For 20 years, the team behind NLAAD has worked tirelessly to create awareness year after year in the Latino communities,...
Category: NLAAD Campaign
NLAAD: HIV in Hispanics/Latinos During the year 2020
Credits: this summary of the impact of HIV among Hispanics/Latinos by gender, sexual orientation and age group has been prepared by Luis A. Mares, LMSW GENERAL Hispanic/Latino Americans in the United States have to deal with a variety of social determinants of health that put them in a precarious position when it comes to...
Registration of NLAAD events is open, and HIV testing kits request form available for NLAAD 2022 campaign.
Creating awareness in the Hispanic/Latino Community through events nationwide has been the main goal of NLAAD through the years. Now that restrictions due to COVID-19 are not as strict as previous years we hope we can organize live events again. We won’t dismay in our efforts to end the HIV epidemic, and to deliver information and...
Registrations Open for NLAAD Webinar Series 2022
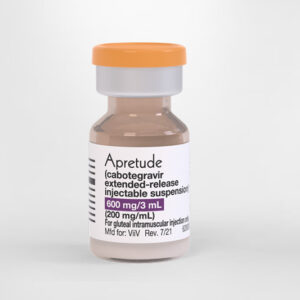
Tuesday October 4th, 3pm “A different PrEP Option: A Presentation for PrEP Professionals” Apretude is the first and only long acting injectable PrEP for reducing the risk of getting HIV. Join us to this presentation given by ViiV laboratories with information about this new important medical advance in the HIV field. [Registration link] Wednesday...
Launching of National Latino AIDS Awareness Day 2022: “You Choose!”
New York, September 15, 2022- October 15th is National Latinx AIDS Awareness Day (NLAAD). NLAAD’s campaign brings together Hispanic/Latinx communities and service providers, and organizations providing services to Hispanic/Latinx throughout the U.S. and territories under one theme. This year’s theme “You Choose!” informs us about new developments in the scientific field of HIV, that provide us...
U.S. FDA Approves CABENUVA for Use Every Two Months, Expanding the Label of the First and Only Long-Acting HIV Treatment
TITUSVILLE, N.J., February 1, 2022 – The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the U.S. Food and Drug Administration (FDA) has approved an expanded label for CABENUVA (rilpivirine and cabotegravir) to be administered every two months for the treatment of HIV-1 in virologically suppressed adults (HIV-1 RNA less than 50 copies per...
ViiV Healthcare announces US FDA approval of Apretude, the first and only long-acting injectable option for HIV prevention
London, 21 December 2021 – ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (GSK), with Pfizer Inc. (Pfizer) and Shionogi Limited (Shionogi) as shareholders, today announced that the US Food and Drug Administration (FDA) approved Apretude, the first and only long-acting injectable pre-exposure prophylaxis (PrEP) option to reduce the risk of sexually...